Iron limitation in the ocean
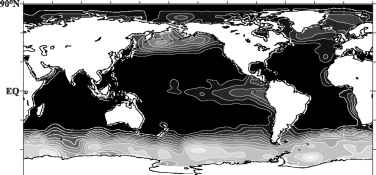
A long-standing puzzle in oceanography has been why phytoplankton do not always fully utilise the nitrate that is supplied to them by the circulation of the ocean and thus why the biological pump does not always work at its maximum possible rate. In certain areas of the world ocean and the Southern Ocean in particular, high concentrations of NOg~ remain in surface waters (Fig. 3). A similar situation prevails for PO3- (not shown). Despite the availability of NOg~, standing stocks of phytoplankton are relatively low, leading to the designation of such regions as 'High-Nutrient
0 5 10 15 20 25 30
surface ocean nitrate concentration (nmol kg ')
Fig. 3 Global distribution of near-surface (30 m depth) ocean nitrate (NO3 ) concentrations [Conkright et al. (1994)].
Low-Chlorophyll' (HNLC). Although physical conditions (such as low light levels) and the intensity of grazing by microscopic marine animals ('zooplankton') can help account for the HNLC condition they are not sufficient explanations on their own.
In the late 1980s came the idea that insufficient availability of iron might also restrict phytoplankton growth [Martin and Fitzwater (1998)]. Iron is essential for enzymatic activities associated with photosynthesis. Laboratory experiments demonstrated that the addition of Fe to HNLC water samples almost invariably stimulated phytoplankton growth and increased NO- uptake. However, because the in vitro environment differs in a number of crucial respects from that of the ocean, the results of these small-scale experiments could not unambiguously tell us what was happening out in the open ocean.
A methodology for carrying out Fe fertilisation of the ocean was devised [Watson et al. (1991)], involving the dispersal of dissolved Fe from a ship whilst simultaneously marking the resulting 'patch' of enhanced Fe with an easily measurable label such as the inert tracersulphur hexafluoride(SF6)。铁释放后,crisscro补丁ssed, and observations made both within and outside the patch (as defined by the presence or absence of SF6 in the water, respectively). The water outside acts as a 'control' on any changes measured in the Fe-enriched patch.
One such experiment was carried out in February of 1999 in the Southern Ocean — the 'Southern Ocean Iron RElease Experiment' (SOIREE) [Boyd et al. (2000)]. As hypothesised, the phytoplankton responded to the addition of Fe with a strong increase in the concentration of chlorophyll a within the fertilised patch but not outside it. (Chlorophyll a is a phy-toplankton photosynthetic pigment whose concentration can be taken as a rough indicator of cell density.) In SOIREE, the impact of iron fertilisation was so striking that the results of the experiment were visible from space! Six weeks after the initial Fe release, gaps in the cloud cover allowed the remote sensing of surface ocean optical properties with the satellite image (Fig. 4) showing a 'bloom' of enhanced chlorophyll concentrations compared to the surrounding waters.
Continue reading here:Volcanically triggered global warming
Was this article helpful?
Readers' Questions
-
winta6 days ago
- Reply