Defoliators
Strength in Simplicity
1. INTRODUCTION
岛屿是深刻有趣的生态系统hich to examine evolutionary patterns and processes. Their isolation and simplicity provide natural 'Ecotrons' in which to seek an understanding of the role of biological diversity in the functioning of ecosystems. Insular biotas are typically relictual, depauperate and disharmonic (Paulay 1994) and hold significantly less biodiversity than equivalent mainland habitats (Whittaker 1998). The MacArthur and Wilson (1967) theory of island biogeography also suggests that the continuous immigration and extinction of species on islands results in a dynamic equilibrium of constant species turnover. Not surprisingly it has become axiomatic to represent island ecosystems as simple, vulnerable, and particularly susceptible to the loss of endemic biodiversity following invasion by continental biota (MacArthur & Wilson 1967, Paulay 1994, Whittaker 1998, Primack 2002). Although empirically not well supported (Wilson 2001), the appealing logic of the equilibrium theory persists and has even been applied to insect: plant interactions (Janzen 1968, Opler 1974, Feeny 1976). However, the spectacular divergence of island biota has been recognized since Wallace (1858) and Darwin (1859). Collectively, islands hold greater biodiversity than the continents and the disproportionate endemicity of island flora, and co-evolved associate invertebrate fauna, suggests evolutionary time-scales of stability or predictability.
Coevolutionand predictability are at the basis of our current understanding of insect: plant interaction (Cornell & Hawkins 2003). In explanations ofsecondary plant compoundsand insect host-specificity, Dethier (1954) and Erlich and Raven (1964) argue for a dynamic coevolution where, in reciprocal adaptations, plants are selected to produce novel compounds as a defense against herbivores, while herbivores develop defense-catabolizing mechanisms in response. Feeny's (1975, 1976) concept of host apparency attempted to classify theseplant secondary compoundsand to explain their floristic distribution with regard to the community
T. D. Paine (ed.), InvasiveForest Insects, Introduced Forest Trees, and Altered Ecosystems, 1-13. © 2006 Springer.
status of the plant. Southwood (1961) had already demonstrated a positive correlation between the geographic range, or the predictability of host plant occurrence, and the diversity of associated herbivores for the trees of Britain. Feeny contended that plants that are more 'apparent', through abundance orlongevity, have to adapt to this greater diversity of herbivores. He reasoned that woody climax species, which often occur as virtually monospecific communities, should employ 'quantitative defenses', such as tannins and leaf toughness, to provide protection against a wide range of herbivores. These non-specific deterrents provide a non-negotiable defense, which reduce foliage digestibility in proportion to their consumption. In contrast, he considered that less apparent plants may escape herbivory in space and time, or be 'qualitatively defended' by relatively low concentrations of unique toxins, which may only be countered by specialist herbivores. The melding of the coevolution and apparency hypotheses was taken a step further by Levin (1975), who predicted that plant resistance to invertebrate herbivores would best be sought at the center, the area of greatest apparency, of a plant's geographic range. However, the geographic variation in plant defenses against herbivores has not been well addressed empirically.
Janzen (1975), Levin (1976) and later Moody (1978) argued that a negative correlation in the latitudinal distribution of alkaloids and other secondary plant compounds was a response to grazing pressure. The gypsy moth (Lymantria dispar) bioassays of Miller and Hanson (1989) produced evidence thattropical treesare better defended than temperate counterparts and literature reviews (Coley & Barone 1996, Dyer & Coley 2002) support that conclusion. Carlquist (1974) described the Hawaiian Islands as being exceptionally poor in poisonous plants and suggested that a relaxation of herbivore pressure on islands led to the loss of defensive chemicals in island flora. The loss of cyanogenesis in plants of the Galapagos Islands has also been interpreted in terms of reduced herbivore pressure (Adersen et al., 1988), and a general loss of defenses against vertebrate herbivores in insular plants is reported by Bowen and van Vuren (1997).
The collective wisdom from biogeography, coevolution, apparency and biodiversity hypotheses would predict that simple, temperate, insular flora should exhibit an inherent vulnerability to invading continental,r-selectedgeneralists. Why is it then that the temperate, insular, New Zealand forest flora is apparently resistant to invasive, polyphagous, continental Macrolepidoptera?
2. THE NEW ZEALAND EXPERIENCE WITH DEFOLIATORS
Some would consider New Zealand to be more than an island. However, 65MY of isolation and periods of glaciation and marinetransgression, which at times restricted the endemics to tenuous refugia, have limited the present day endemic biota to a state considered depauperate even by island standards (Lawton 2000, Rosenzweig pers. comm.). Watson (2002) would argue that the difference in character between a biota built through vicariance, and one built by long-distance dispersal, would be lost over such a period. However, the oceanic debate may be of little consequence, for the thesis offered here appears to apply equally to mainland islands, or any plant population that is isolated, even when surrounded by congenerics.
2.1. Bioassays with invasive Lepidoptera
Lymantriids are not present in the New Zealand endemic invertebrate fauna and a rash of establishments and interceptions of lymantriids (Orgyia thyellina, Teia anartoides, Lymantria dispar) and the invasive arctiid, Hyphantria cunea, over the last decade should have resulted in the easy naturalization of those forest defoliators. Considerable eradication programs were put in place to prevent this (Hosking et al., 2003) and were supported by coincidental bioassays of the invaders to assess the risk they posed to the naturalized and endemic flora. All of the bioassays followed the methods of Matsuki et al. (2001). In no-choice randomized block trials, parameters of larval mortality and development of neonates were recorded till death or pupation.
2.1.1. Differences between continental and island plants
The bioassays (Kay 2002, 2003, 2004; Kay et al., 2000; Matsuki et al., 2001; Hosking et al., 2003) showed a surprising degree of resistance to these defoliators within the endemic flora. Furthermore, the New Zealand representatives of the Southern Hemisphere climax forest genus, Nothofagus (Fagales: Nothofagaceae), were largely unpalatable to these exotic defoliators.
Nothofagus species are the long-lived, climax dominants of the endemic temperate forests of the Southern Hemisphere and typically occur as 'predictable' monospecific forests. The greatest species diversity and geographic range of the genus occurs in South America. In an apparent paradox, the bioassays revealed that the continental South American species of Nothofagus, which are subject to a far greater diversity of invertebrates than the New Zealand representatives (McQuillan 1993; Ogden et al., 1996; Veblen et al., 1996), were generally more palatable than those of New Zealand. Some South American Nothofagus were more palatable to the assaying Lepidoptera than their primary Northern Hemisphere host plants!
2.1.2. Latitude and Nothofagus defense
If plant defenses are negatively correlated with latitude, one would expectlower latitudeNothofagus to be better defended thanhigher latitude物种。这不是假山毛榉的证实of South America. The most northern species N. obliqua (33°-41°S) is demonstrably the most palatable in all bioassays. However, N. alessandri (35-36°S), a tiny (350 ha) relictual population at the same latitude is the least palatable species (Russell et al., 2000; Kay 2002; Matsuki et al., 2001). Furthermore, N. obliqua has the greatest number of associated invertebrates and N. alessandri the least (McQuillan 1993). The Australian N. moorei (28-32°S) is the most northern of the temperate Nothofagus, but is also of very limited distribution. It is the least palatable of the Australian species (Kay 2002). Nothofagus alessandri and N. moorei could be considered to be mainland islands of Nothofagus, and it would appear that the limited geographic range of a species is just as important for plant defense on mainlands as it is on islands. It would also appear that area is more important than latitude in determining plant defense.
A compounding effect of latitude may be demonstrated in bioassays of provenances of N. truncata, New Zealand's least palatable beech species. Nothofagus truncata is the least common of the New Zealand beeches and has a small (ca 40ha) southern population at Haast (44°S), isolated by some 260 km to the south of the nearest substantial population of the species (Mark & Lee 1985). In a bioassay with T. anartoides and two provenances of N. truncata, the southernmost, Haast provenance proved totally resistant (100% larval mortality) whereas larvae on some replicates of the Mangorewa (38°S) provenance, close to the center of the geographic range of N. truncata, survived to produce male pupae (Fig. 1).

25 days
Figure 1. The survivorship of Teia anartoides fed on Haast (solid) and Mangorewa (hollow) provenances ofNothofagus truncata.
25 days
Figure 1. The survivorship of Teia anartoides fed on Haast (solid) and Mangorewa (hollow) provenances ofNothofagus truncata.
2.1.3. A reciprocal test
Pinus radiata is the main plantation species in New Zealand. Pinus is almost exclusively confined to the Northern Hemisphere and the geographic range of
P.radiata is limited to three discrete mainland and two island populations, along the coast of California.
In a bioassay, foliage from the natural populations of P. radiata, grown in a common garden in New Zealand, was fed to the New Zealand indigenous geometrid, Pseudocoremia suavis. Larval growth rate was poorest on the two island provenances of P. radiata and there was a positive correlation between the growth rate and the geographic range of the individual Californian provenances (Fig. 2). The island provenances were the least palatable, but also the provenances of lowest latitude. The provenance with the smallest geographic range (Cedros Island) was the least palatable. Interestingly the New Zealand landrace, the first generation commercially selected for growth, was the most palatable.
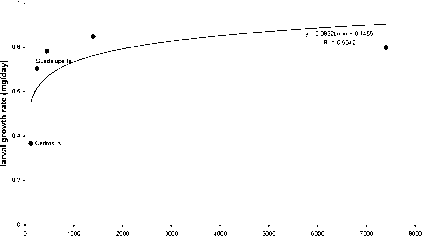
geographic range (ha)
Figure 2. The growth rate o/Pseudocoremia suavis fed on Pinus radiata from geographically distinct natural populations.
geographic range (ha)
Figure 2. The growth rate o/Pseudocoremia suavis fed on Pinus radiata from geographically distinct natural populations.
3. AN EXPLANATION OF THE NEW ZEALAND EXPERIENCE
岛上资源分配(IRA)假说(Kay & Wratten 2004) was developed as an explanation of the apparent resistance of the New Zealand flora to novel defoliators. The basis for the IRA hypothesis is a redefinition of the fundamental ecological principle of the species: area relationship. Species diversity increases with area, but the relationship is more pronounced at higher trophic levels (Holt 1996). Habitat fragmentation studies (Schoener 1989, Kreuss & Tscharntke 1994, Lawton 2000, Komonen et al., 2000) and trophic level patterns in spatially discrete ecosystems (Schoenly et al., 1991; Holt 1996) show that the trophic components of invertebrate communities do not change uniformly with area. Rather, during habitat fragmentation, the higher trophic levels are lost prematurely or disproportionately, or cannot be maintained in small habitats. In redefining the species: area concept, the IRA hypothesis contends that islands, or similarly geographically constrained ecosystems, support lower biodiversity, have fewer trophic levels, and consequently have a lesser top-down regulation of herbivores by natural enemies. The hypothesis posits that if plant fitness is influenced by herbivores, then, when top-down regulation of herbivores is weak because of a lack of trophic complexity, plants that cannot escape in space or time will be selected to allocate resources to bottom-up defenses. If trophic diversity drives resource allocation for plant defense, the lower biodiversity typical of higher latitudes may compound the species: area effect.
The breakup of theGondwanancontinent represents a fragmentation of the Nothofagus ecosystem on a macroecological scale, over evolutionary time. The remnants of that temperate ecosystem exist in Australia, New Zealand and South America. The isolation and inundation of the New Zealand landmass has resulted in an island-like depauperate biota lacking trophic complexity. On the continents similar climatic events have marginalized some species so that they resemble mainland islands. Bioassays show that these geographically constrained species are well defended against defoliators.
4. DISCUSSION
4.1. The IRA hypothesis
It is generally accepted that, in response to herbivore selection pressure, plants have evolved a multitude of physical, chemical, phenological and symbiotic strategies which provide a bottom-up limitation on the plant material available to defoliators (Edwards & Wratten 1980). These strategies may be complemented by the actions of natural enemies, which effect a top-down regulation of herbivores (Hairston et al., 1960). This regulatory plurality is now considered to exist in most systems (Hunter & Price 1992), but it is empirically difficult to demonstrate the relative roles of each (Walker & Jones 2001).
With the trend to reductionist studies there seems to have been few empirical attempts to put plant defense strategies into a community or ecological context. The majority of literature on the interaction between insects and plants neglects the concomitant interaction with other trophic levels. The task may be impossible empirically. Even the modelling of such interaction is seen as daunting in its complexity (Holt & Loreau 2001) and yet unrealistic if simplified (Polis 1991). The 'Green World' hypothesis (Hairston et al., 1960) argues that the necessity for plant defenses is minimal because of the top-down regulation of herbivores that is effected by natural enemies. Conversely, not all plant material is available to herbivores. Plant defenses may provide a bottom-up limitation of herbivore populations (White 1978). It would appear logical that, if insect herbivory does influence plant fitness, then, when a robust top-down regulation of herbivore populations by natural enemies is reduced or absent over evolutionary time, plants that cannot escape in space or time should be selected to allocate resources to a 'bottom-up' defense. The role of biodiversity in ecosystem stability is a hotly debated issue (Kinzig et al., 2001), but the IRA hypothesis contends that ecosystem stability may be achieved through a reconciliation of top-down and bottom-up processes mediated primarily by biodiversity via habitat area. This appears counter-intuitive only because of long established, but poorly supported, hypotheses of biogeography and plant apparency.
Feeny's classification of defensive plant compounds has become blurred. Feeny (1975) himself argued for both quantitative and qualitative strategies in climax plant species and the comparative palatability of British trees has not been convincingly demonstrated (Edwards et al., 1986). More recently qualitative defenses have been shown to act in a dose-dependent quantitative manner (Lindroth & Hemming 1990) and the role of tannins in plants has been the subject of numerous studies which show a great and varied array of responses. Needless to say, bioassays vary, tannins are an extremely variable group of compounds and the apparency hypothesis persists (Coley 1983; Forkner et al., 2004). The IRA hypothesis would conclude that the polarity of the apparency hypothesis should be reversed. Widely dispersed plants are protected from the excesses of herbivores by an accompanying complex trophic web. Geographically constrained plants need inherent defenses against herbivores and the need increases as habitat area decreases i.e. an 'unapparency' hypothesis of plant defense might be more appropriate.
推论的可见性(芬尼1976)和coevolution hypotheses posit that isolated plants with non-evolving defensive compounds would be easily overcome through herbivore selection pressure, and that related plants should have similar secondary chemistries. The ecography of secondary plant chemicals has not been well addressed however, Bohm's (1998) limited review of insular secondary plant chemistry shows that island plant chemical profiles are somewhat ambivalent. They may be similar, simpler or enriched, when compared to the profiles of equivalent continental species. The New Zealand flora has a high (80%) degree of endemism (Allan 1982) which may provide a novelty that would deter the most catholic of feeders. However, in numerous well-documented instances New Zealand plants have richer arrays of compounds than their continental counterparts. Plant phylogeny does play a part in chemical profiles, but for Nothofagus the resistance to defoliators occurs within and between the three temperate subspecies and landmasses, which suggests that factors other than phylogeny influence chemical expression.
Levin's (1975) claim that resistance to herbivores should be found at the center of a plant's geographic range because of the greater diversity of herbivores, neglects the influence of natural enemies which would be expected to accompany the herbivores. The IRA hypothesis, which includes community trophic complexity, indicates that resistance should be sought in isolated populations or at the edges of populations. The expansion and contraction of plant ranges due to climate or competition will produce 'marginal populations' sensu Stern and Roche (1974), which act as 'mainland' islands. These isolated populations are subject to the selection extremes of climate and competition for the species. It appears, as has been demonstrated for Nothofagus and Pinus radiata, that these marginal populations are also selected for defense against herbivores. A similar demonstration of resistance at the edge of populations has been seen in the interaction between the white pine weevil and spruce in Canada (Alfaro et al., 1999).
Biologically active compounds in plant leaves may well permeate through the food web, unless catabolized by the plant at leaf senescence. Interestingly, Wardle et al. (1997) found that the accumulation of secondary plant compounds in litter increased with the inverse area of islands of high latitude Scandinavian lakes. Area is debatably the best predictor of biodiversity (Rosenzweig 1995, 1997). If biodiversity is the mediator in the deployment of plant defenses, high latitude and high altitude populations of a species may also reveal resistant plants.
4.2. Latitude: are the temperate and tropical forests so different?
Latitudinal gradients in biodiversity are some of the oldest observations in published ecology (Wallace 1878). The evidence from the Nothofagus forests shows a marked divergence from the commonly advocated ideas of latitude and the deployment of herbivore defense. For the lower latitude species, N. obliqua, N. alessandri and N. moorei, area, or the extent of a plant's geographic range, appeared to have a much greater influence than latitude. For N. truncata and P. radiata the influence of latitude may be compounding the low area: biodiversity effect.
Tropical forests differ markedly from those of the boreal or temperate regions in that they consist of a vast array of plant species rather than the more mono-specific forests of the higher latitudes. Is it that in such a diverse tropical environment individual plants are 'islands' in their own right, sensu Janzen (1968) and Opler (1974), and have to defend themselves despite the great overall diversity of natural enemies? The spatial complexity of the tropical forest may make regulation of herbivores by natural enemies more difficult. Could the bottom-up limitation of herbivores that appears to operate on oceanic and mainland islands be extrapolated to individual trees in the tropics?
Host specialization is generally construed as a result of coevolution with qualitative chemical defenses. Dobzhansky (1950), Pianka (1966) and MacArthur and Wilson (1967) speculated that the greater diversity of herbivores in the tropics resulted from a greater degree of host specialization, which allowed greater species packing. Specialization has been quoted as a feature of tropical invertebrate herbivores (Dyer & Coley 2002), but it is also a feature of New Zealand invertebrates (Dugdale 1975). Is the concentration of secondary plant compounds in the tropics apparent only because previous authors have not considered the influence of area? It is always easier to assay a common representative of a temperate genus or family, than a rare one and bioassay results of temperate species are often extrapolated to include congenerics (Barbosa & Krischik 1987). The results from the study by Miller and Hanson (1989) using L. dispar bioassays and tropical plants are no better than temperate New Zealand examples using the same assay.
4.3. Growth-rate and plant defense
一些作者假定资源紧密相联的cation to defense is greatest in slow-growing plant species in resource-poor environments (Janzen 1974; Coley et al., 1985; Coley 1988; Price 1991; Herms & Mattson 1992). In New Zealand the contrast between the slow growth rate of endemic forest trees and the spectacular growth rate of exotics was recognized by early foresters. Although New Zealand was almost totally covered by forest at the time of European settlement, the slow growth of endemic timber species necessitated a switch to exotic forest species within 100 years to create a sustainable forest industry. That exotics grow better in New Zealand than in their homelands suggests that New Zealand endemics are not constrained by resources. Is their slow growth rate a result of allocation of resources to defense rather than a need for a defense because they grow so slowly? There is little in the way of comparative growth data for Nothofagus. However, New Zealand species failed to thrive in the experimental plantings of the genus in UK, whereas the South American species N. obliqua (the most palatable species) has potential for plantation forestry there (Tuley 1980). It is also interesting to note that New Zealand commercial selections of Pinus radiata grow more rapidly than the original Californian populations, but are considerably more palatable to invertebrate defoliators. The artificial selection for growth appears to have been at the expense of resource allocation to defense.
5. CONCLUSION
The complexity of food web dynamics has restricted the empirical synthesis of the ecological forces that shape species evolution within component communities. The truth of the matter is, that very few insect: plant studies have included tritrophic interactions, other than the effect of plant defense chemistry on the fitness of natural enemies. A plethora of hypotheses has been developed for specific interactions but no over-arching hypothesis, that is empirically testable, suits all. The IRA hypothesis is testable and offers a 'red-shift' proof that biodiversity determines ecosystem function, without reverting to speciously simplified models or expensive anthropogenic ecotrons. Contrary to accepted wisdom, island forests appear resistant to invading continental defoliators. A marked difference in the deployment of defense against invertebrate herbivores has been demonstrated between congeneric and conspecific plants in essentially identical habitats of differing area. For insular forests there appears to be an inherent strength in the simplicity of island communities. Area is the primary determinant of biodiversity and trophic complexity. The IRA interpretation of the interaction between climax forest and defoliators refutes established hypotheses. Instead it posits that for any forest the interaction with defoliators is determined by the relative strengths of top-down and bottom-up forces system, which are mediated by habitat area, and that the habitat area may be oceanic or continental, and of temperate or tropical species.
New Zealand Forest Research Institute
Private Bag 3020
Rotorua
New Zealand
Ph +64 7 343 5500
e-mail[email protected]
6. REFERENCES
Adersen, A., Adersen, H., & Brimer, L. (1988). Cyanogenic constituents in plants from the Galapagos
Islands. Biochemistry Systematics and Ecology, 16, 65-77. Alfaro, E. Tomlin, J.A. Borden & K. Lewis. (1999). Interaction of the white pine weevil and its hosts: arguments for coevolution. pp. 31-39, In F. Lieutier, W.J. Mattson, M.R. Wagner (Eds.), Physiology and genetics of tree-phytophage interactions. Proceedings of an IUFRO meeting, Gujan,France. Aug. 31-Sept. 5, 1997. INRA, Paris. 374 pp. Allan, H. H. (1982). Flora of New Zealand. Vol.1. Wellington : Hasselberg, Government Printer, Barbosa, P., & Krischik, V. A. (1987). Influence of alkaloids on feeding preference of eastern deciduous forest trees by the Gypsy moth, Lymantria dispar. American Naturalist, 130, 53-69. Bohm, B. A. (1998). General evolutionary patterns and processes on oceanic islands. In T.F. Stuessy &
M. Ono (Eds.), Evolution and speciation of island plants. Cambridge University Press. Bowen, E. & van Vuren, D. (1997). Insular endemic plants lack defences against herbivores.
Conservation Biology, 11, 1249-1254. Carlquist, S. (1974). Island biology. New York: Columbia University Press.
Coley, P.D. (1983). Herbivory and defensive characteristics of tree species in a lowland tropical forest.
Ecological Monographs, 53, 209-233. Coley, P. D., Bryant, J. P. & Chapin, F. S. (1985). Resource availability and plant antiherbivore defense.
Science, 230, 895-899.
Coley, P. D. (1988). Effects of plant growth rate and leaf lifetime on the amount and type of antiherbivore defense. Oecologia, 74, 531- 536. Coley, P. D. & Barone, J. A. (1996). Herbivory and plant defences in tropical forests. Annual Review of
Ecology & Systematics, 27, 305-335. Cornell, H. V. & Hawkins, B. A. (2003). Herbivore response to plant secondary compounds: A test of phytochemical coevolution theory. American Naturalist, 161; 508-522. Darwin, C. (1859). On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. London: John Murray Dethier, V. G. (1954). Evolution of feeding preferences in phytophagous insects. Evolution, 8, Dobzhansky, T. (1950). Evolution in the tropics. American Scientist, 38, 209-221
Dugdale, J. S. (1975). The insects in relation to plants. In G. Kuschel, ed. Biogeography and ecology in
New Zealand. Monographiae Biologicae 27, The Hague: Junk, Dyer, L. A. & Coley, P. D. (2002). Tritrophic interactions in tropical versus temperate communities. In T.
Tscharntke & B. A. Hawkins (Eds.), Multitrophic level interactions. Cambridge University Press Edwards, P. J. & Wratten, S. D. (1980). Ecology of insect-plant interactions. Studies in Biology 121. London: Edward Arnold
Edwards, P. J., Wratten, S. D. & Greenwood, S. (1986). Palatability of British trees to insects:
constitutive and induced defences. Oecologia, 69, 316-319. Erlich, P. R. & Raven, P. H. (1964). Butterflies and plants: a study in coevolution. Evolution, 18, 586608.
Feeny, P. (1975). Biochemical coevolution between plants and their insect herbivores. In L.E. Gilbert &
P.H. Raven (Eds.), Coevolution of animals and plants. Austin: University of Texas Press. Feeny, P. (1976). Plant apparency and chemical defence. Recent Advances in Phytochemistry, 10, 1-40. Forkner, R. E., Marquis, R. J. & Lill, J. T. (2004). Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecological Entomology, 29, 174-187
Futuyma, D. J. & Mitter, C. (1997). Insect-plant interactions: the evolution of component communities. In: Silvertown, J.; Franco, M.; Harper, J. L. (Eds.), Plant Life Histories (pp 253-264). UK: Cambridge University Press.
Hairston, N. G., Smith, F. E. & Slobodkin L. B. (1960). Community structure, population control and competition. American Naturalist, 94, 421-425.
Herms. D. A. & Mattson, W. J. (1992). The dilemma of plants to grow or defend. Quarterly Review of Biology, 67, 283-335.
Holt, R. D. (1996). Food webs in space: an island biogeographic perspective. In G.A.Polis and K.O. Winemiller. (Eds.), Food Webs. Integration of patterns and Dynamics (pp. 312-323). New York: Chapman and Hall,
Holt, R. D., Lawton, J. H., Polis, G. A., & Martinez, N. D. 1999. Trophic rank and the species-area relationship. Ecology, 80, 1495-1504.
霍尔特,r·d·& Loreau M。,(2001)。生物多样性和ecosystem functioning: the role of trophic interactions and the importance of system openness. In A. P. Kinzig, S. W. Pacala & D. Tilman (Eds.), The functional consequences of biodiversity: empirical progress and theoretical extensions (pp. 246262). Princeton: Princeton University Press,
Hosking, G.P., Clearwater, J., Handiside, J., Kay, M., Ray, J. & Simmons, N. (2003). Tussock moth eradication - a success story from New Zealand. International Journal of Pest Management, 49, 1724.
猎人,m . D。&价格p w(1992)。玩啧es and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology, 73, 724-732.
Janzen, D. H. (1968). Host plants as islands in evolutionary and contemporary time. American Naturalist, 102, 592-595.
Janzen, D. H. (1974). Tropical blackwater rivers, animals andmast fruitingby the Dipterocarpaceae.
Biotropica, 6, 69-103.
Janzen, D. H. (1975). The ecology of plants in the tropics. Studies in Biology. 58, London: Edward Arnold.
Kay, M. K. (2002). The investigation of the suitability of the New Zealand forest flora for the reproduction and development of Lymantriid species. Unpubl. MAFResearch Report, 42pp.
Kay, M.K. (2003). Macroecology and the prediction of invasive invertebrate guilds. Goldson, S & Suckling, M. (Eds.), Defending the Green Oasis: New Zealand Biosecurity and Science. Proceedings of the Plant Protection Society NZ., Biosecurity Symposium, Rotorua, 2003
Kay, M. K. (2004). An assessment of the risk to the New Zealand flora posed by Japanese strains of the Gypsy moth and the Fall web-worm . Unpubl. MAF Research Report, 29pp.
Kay, M., Matsuki, M., Serin, J. & Scott, J.K. (2000). A risk assessment of the Asian gypsy moth to key elements of the New Zealand flora. Unpbl. MAF Research Report 82pp.
Kay, M. K. & Wratten, S. D. (2004). Ecosystem function and the prediction of tree resistance to defoliators. . In N. Kamata (Ed.), Proceedings International Symposium of the Kanazawa University 21st Century COEProgram., 11.-15.11.2003, Kanzawa University, Japan, in press
Kinzig, A. P., Pacala, S. W. & Tilman, D. (Eds.) (2001). The functional consequences of biodiversity. Monograph of Population Biology 33, Princeton: Princeton University Press.
Komonen, A., Penttila, R., Lindgren, M. & Hanski, I. (2000). Forest fragmentation truncates a food chain based on an old-growth forest bracket fungus. Oikos, 90, 119-126.
Kreuss, a & Tscharntke t(1994)。栖息地fragmentation, species loss and biological control. Science, 264, 1581-1584.
Lawton, J. H. (2000). Community ecology in a changing world. Excellence in Ecology 11, Germany: Ecology Institute.
Levin, D. A. (1975). Pest pressure and recombination systems in plants. American Naturalist, 109, 437451
Levin D. A. (1976). Alkaloid-bearing plants: an ecogeographical perspective. American Naturalist, 110, 261-84
Lindroth, R.L., & J.D.C. Hemming. (1990). Responses of the gypsy moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environmental Entomology, 19, 842-847.
MacArthur, R. H. & Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton: Princeton University Press.
Mark, A. F. & Lee, W. G. (1985). Ecology of hard beech (Nothofagus truncata) in southern outlier stands in the Haast ecological district, South Westland, New Zealand. New Zealand Journal of Ecology, 8, 97-115
Matsuki, M., Kay, M., Serin, J., Floyd, R. & Scott, J. K. (2001). Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: effects of climatic conditions and suitability of native plants. Agricultural and Forest Entomology, 3, 305-320. McQuillan, P. B. (1993). Nothofagus (Fagaceae) and its invertebrate fauna- an overview and preliminary synthesis. Biological Journal of the Linnean Society, 49, 317-354. Miller, J. C. & Hanson, P. E. (1989). Laboratory feeding tests on the development of gypsy moth larvae with reference to plant taxa and allelochemicals. Bulletin of the Agricultural Experimental Station, Oregon State University 674, 1-63 Moody, S. (1978). Latitude,continental driftand the percentage of alkaloid-bearing plants in floras.
American Naturalist, 113, 965-72 Ogden, J., Stewart, G. H. & Allen, R. B. (1996). Ecology of New Zealand Nothofagus forests. In T. T. Veblen, R.S. Hill & J Read (Eds.), The Ecology and Biogeography of Nothofagus Forests (pp 2582). Hew Haven: Yale University Press. Opler, P. A. (1974). Oaks as evolutionary islands for leaf-mining insects. American Science, 62, 67-73 Paulay, G. (1994). Biodiversity on oceanic islands: its origin and extinction. American Zoologist, 34, 134144
Pianka, E. R. (1966). Latitudinal gradients in species diversity: a review of concepts. American Naturalist, 100, 33-46
Polis, G. A. (1991). Complex trophic interactionsin deserts: an empirical critique of food web theory.
American Naturalist, 138, 123-155 Price, P. W. (1991). The plant vigor hypothesis and herbivore attack. Oikos, 62, 244-251 Primack, R. (2002). Essentials of conservation biology. 3rd ed. Massachusetts: Sinauer Associates Inc. Rhoades, D.F. & Cates, R.G. (1976). Toward a general theory of plant antiherbivore chemistry. Recent
Advances in Phytochemistry, 10, 168-213 Rosenzweig, M. L. (1995). Species Diversity in Space and Time. UK: Cambridge University Press. Rosenzweig, M. L. (1997). Species diversity and latitudes: listening to area's signal. Oikos, 80, 172-176 Russell, G. B., Bowers, W. S., Keesing, V., Niemeyer, H. M., Sevenet, T., Vasanthaverni, S. & Wratten, S. D. (2000). Patterns of bioactivity and herbivory on Nothofagus species from Chile and New Zealand. Journal of Chemical Ecology, 26(1), 41-56. Schoener, T. W. (1989). Food webs from the small to the large. Ecology, 70, 1559-89. Schoenly, K., Beaver, R. A. & Heumier, T. A. (1991). On the trophic relations of insects: a food-web approach. American Naturalist, 137, 597-638. Southwood, T. R. E. (1961). The number of species of insect associated with various trees. Journal of Animal Ecology, 30, 1-8.
Stern, K. & Roche, L. (1974). Genetics of forest ecosystems. Ecological Studies 6. New York: SpringerVerlag,
Strong, D. R. (1992). Are all trophic cascades wet? Differentiation and donor-control in speciose ecosystems. Ecology, 73 (3), 747-754. Strong, D. R., Lawton J. H. & Southwood, R. (1984). Insects on Plants: Community patterns and mechanisms. Oxford: Blackwell Scientific Publications. Tuley, G. (1980). Nothofagus in Britain. For. Comm. Forest Record 122
Veblen, T. T., Donso, C., Kitzberger, T. & Rebertus, A. J. (1996). Ecology of Southern Chile and Argentina Nothofagus forests. In T. T. Veblen, R. S. Hill, & J. Read (Eds.), The Ecology and Biogeography of Nothofagus Forests, (pp 293-353). Hew Haven: Yale University Press. Walker, M. and Jones, T. H. 2001. Relative roles of top-down and bottom-up forces in terrestrial tri-
trophic plant-insect-natural enemy systems. Oikos, 93, 177-187. Wallace A. R. (1855). On the law which has regulated the introduction of new species. Annual Magazine of Natural History, 16, 184-196. Wallace, A. R. (1878). Tropical nature and other essays. New York: MacMillan.
Wardle, D. A., Zackrisson, O., Hornberg, G., & Gallet, C. (1997). The influence of island area on ecosystem properties. Science 277, 1296-1299. Watson D. M. (2002). A conceptual framework for the study of species composition in islands, fragments and other patchy habitats. Journal of Biogeography, 29, 823-34.
White, T. C. R. (1978). The importance of a relativeshortage of foodin animal ecology. Oecologia, 33, 71-86
Wilson, E. O. (2001). Preface to Edition 2. The Theory of Island Biogeography. MacArthur, R. H. &
Wilson, E. O. Princeton: Princeton University Press. Whittaker, R. J. (1998). Island biogeography. Oxford.
This page intentionally blank
Continue reading here:Native Insects Colonizing Introduced Tree Speciespatterns And Potential Risks
Was this article helpful?