氮同化79表102分子和我ons Providing the Nitrogen Nutrient
Molecule or Ion Organic Form Inorganic Form Readily-Available
Amino acids x
N2 x
NO- x x nium ions are present in the bulk solution,ammonium ionsare used as the nutrient source for nitrogen, and organic-nitrogen compounds are not used.
Bacteria, as a group, use ammonium ions, and most bacteria can utilizenitrite ionsandnitrate ions, but the use of nitrite ions and nitrate ions is somewhat restricted. The use of nitrite ions and nitrate ions as a nutrient source for nitrogen is referred to as assimilatory nitrate or nitrite reduction (Equation 10.1). In order to assimilate nitrogen from these ions, the nitrogen within the ions must be reduced
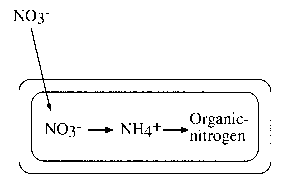
图10.2使用硝酸离子作为营养酸ce for nitrogen. Nitrate ions (NO-) can be reduced biologically to molecular nitrogen (N2) or ammonium ions (NH+). When oxygen is stripped from nitrate ions to form molecular nitrogen as occurs during denitrification, nitrogen is not incorporated or assimilated in cellular material. This form of nitrate reduction is termed dissimilatory nitrate reduction. When ammonium ions (NH+) are no longer available for use by bacteria as a nitrogen nutrient, nitrate ions are then used as the nitrogen nutrient. When nitrate ions are used as the nitrogen nutrient, oxygen is stripped from the nitrogen atom and hydrogen is added to the nitrogen atom. The addition of hydrogen results in the production of ammonium ions and a change in oxidation state for nitrogen from +5 in nitrate ions to -3 in ammonium ions. In this oxidation state or in the form of ammonium ions, nitrogen is assimilated into new cellular material such as amino acid and proteins (organic nitrogen). This form of nitrate reduction is termed ''assimilatory nitrate reduction.''
图10.2使用硝酸离子作为营养酸ce for nitrogen. Nitrate ions (NO-) can be reduced biologically to molecular nitrogen (N2) or ammonium ions (NH+). When oxygen is stripped from nitrate ions to form molecular nitrogen as occurs during denitrification, nitrogen is not incorporated or assimilated in cellular material. This form of nitrate reduction is termed dissimilatory nitrate reduction. When ammonium ions (NH+) are no longer available for use by bacteria as a nitrogen nutrient, nitrate ions are then used as the nitrogen nutrient. When nitrate ions are used as the nitrogen nutrient, oxygen is stripped from the nitrogen atom and hydrogen is added to the nitrogen atom. The addition of hydrogen results in the production of ammonium ions and a change in oxidation state for nitrogen from +5 in nitrate ions to -3 in ammonium ions. In this oxidation state or in the form of ammonium ions, nitrogen is assimilated into new cellular material such as amino acid and proteins (organic nitrogen). This form of nitrate reduction is termed ''assimilatory nitrate reduction.''
TABLE 10.3 Ammonium Ions, Nitrite Ions, and Nitrate Ions As Nutrient
Sources for Nitrogen
TABLE 10.3 Ammonium Ions, Nitrite Ions, and Nitrate Ions As Nutrient
Sources for Nitrogen
Oxidation State |
Nutrient Available for Use due to the |
|
Nutrient Source |
of Nitrogen |
Oxidation State of Nitrogen |
NH+ |
33 |
100% |
NOg |
+3 |
70% |
no3 |
+5 |
30% |
from the +5 oxidation state (nitrate ions) and the +3 oxidation state (nitrite ions) to the —3 oxidation state of nitrogen within the ammonium ion.
NO— ! NO— ! NH+ (10.1)
The reduction in oxidation state for each ion requires cellular energy. Therefore less bacterial growth or MLVSS production is achieved using nitrite ions and nitrate ions as compared to the bacterial growth obtained by using ammonium ions (Table 10.3).
Nitrogen in wastewateris a necessary constituent for the production of new cellular material or MLVSS. Approximately 14% of the nitrogen entering theactivated sludge process isused in cellular growth and reproduction (MLVSS production).
Nitrogen is the major constituent of proteins and nucleic acids (genetic material) of cells, and nitrogen is found in peptidoglycans, the rigid cell wall layer of most bacteria. An additional 10% to 15% of nitrogen entering the activated sludge process is removed in the wasting of excess sludge. The remaining nitrogen is discharged to the receiving water,nitrified, denitrified, or air stripped to the atmosphere.
Nitrification may occur in an aeration tank after adequate,soluble cBODdegradation has occurred and several operational conditions are satisfactory for nitrification to occur. These conditions include the presence of an adequate amount of dissolved oxygen, substrate (ammonium ions or nitrite ions) fornitrifying bacteria, adequate retention time of the wastewater in the aeration tank, and a healthy and active population of nitrifying bacteria. Nitrification more easily occurs in the presence of high MLVSS and high temperatures.
Under favorable operating conditions an activated sludge process may nitrify, because the influent total nitrogen is considerably greater than the MLVSS requirement for the nutrient nitrogen. There are several forms of nitrification that are identified by the amount of ammonium ions, nitrite ions, and nitrate ions produced in the aeration tank or found in the aeration tank or mixed liquor e¿uent (Table 11.1). Testing for these three nitrogenous ions may be performed rapidly. Unless industries discharge wastes that contain nitrite ions or nitrate ions to the sewer system, testing for these two nitrogenous ions in the influent of the aeration tank is seldom performed.
Continue reading here:TABLE 113 Limiting Factors Responsible for Incomplete Nitrification
Was this article helpful?
Readers' Questions
-
margit2 months ago
- Reply