The Wastewater Nitrogen Cycle
Because of the large number of valences or oxidation states of nitrogen, manynitrogenous compoundsexist in the environment and the activated sludge process (Table 9.1). The majority of nitrogen found in the environment exists as molecular nitrogen. Approximately 80% of dry air by volume contains molecular nitrogen and represents an inexhaustible reservoir of this essential element.
Although constituting much less of the biomass than carbon or oxygen, nitrogen is an essential element for all living organisms. It is incorporated in cellular material that is used for growth, enzyme production, and genetic information. However, molecular nitrogen is formed by a triple bond that is very difficult for most organisms to break. Fortunately molecular nitrogen is made available to living organisms when the triple bond is broken by a group of unique bacteria and fixed or converted toammonium ions.
The bacteria that convert molecular nitrogen to ammonium ions arenitrogen-fixing bacteria(Table 9.2). The bacteria may be free-living in the soil around the roots of plants or may grow in a symbiotic relationship in the nodules of the roots of green plants (Figure 9.1).
Nitrogen fixation, namely the conversion of molecular nitrogen to ammonium ions, is achieved by the enzymenitrogenasethat is found only innitrogen-fixing bacteria(Equation 9.1). Prior to the widespread use of nitrogenfertilizersnodule-growing植物或豆类提供土壤nitrogen. Examples of legumes include alfalfa, clover, and soybeans.
Compound |
Oxidation State |
Chemical Name |
NH3 |
33 |
Ammonia |
NH+ |
33 |
Ammonium ion |
NH4OH |
33 |
Aqua ammonia |
NH4HCO3 |
33 |
Ammonium bicarbonate |
N2 |
O |
Molecular nitrogen |
NO3 |
+3 |
|
NO33 |
+5 |
Nitrate ion |
The nodules that develop in the roots of the plant result from an "irritating" action caused by the nitrogen-fixing bacteria. The nodules connect directly to the vascular (circulatory) system of the plant. This connection enables a symbiotic or mutually advantageous relationship between the bacteria and the plant. The bacteria obtain photo-synthetically produced energy directly from the plant, while the plant obtains ammonium ions. The ammonium ions are assimilated into plant material in the form of amino acids and proteins within tissues, roots, and seeds. The plant may be consumed and pass its ammonium ions along the food chain, or the plant may die and decompose. When the plant decomposes, it releases ammonium ions to the soil that can be used by other plants.
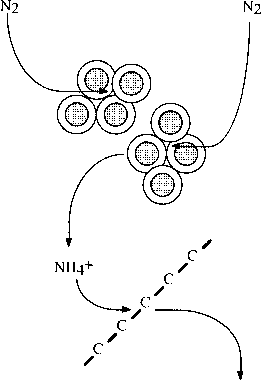
Some algae also can use molecular nitrogen for the production of amino acids and proteins. The algae take the molecular nitrogen from
Type of Nitrogen-Fixing Bacteria |
Genus |
Free-living bacteria |
Arthrobacter |
Azobacter |
|
Azotobacter |
|
Azospirillum |
|
Clostridium |
|
Cyanobacterium |
|
Enterobacter |
|
Symbiotic bacteria |
Frankia |
Rhizobium |
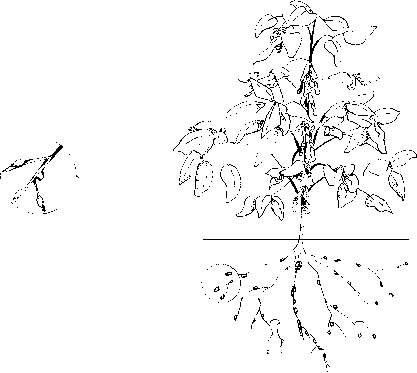
Figure 9.1Root nodulesand nitrogen-fixing bacteria. Bacteria that fix molecular nitrogen (N2) are found in the soil immediately around the roots of plants or in nodules growing on the surface of the roots of plants. These nitrogen-fixing bacteria convert molecular nitrogen to ammonium ions (NH¿). Nitrogen-fixing bacteria that live in the soil are free-living, while nitrogen-fixing bacteria that live in the nodules are symbiotic.
Figure 9.1 Root nodules and nitrogen-fixing bacteria. Bacteria that fix molecular nitrogen (N2) are found in the soil immediately around the roots of plants or in nodules growing on the surface of the roots of plants. These nitrogen-fixing bacteria convert molecular nitrogen to ammonium ions (NH¿). Nitrogen-fixing bacteria that live in the soil are free-living, while nitrogen-fixing bacteria that live in the nodules are symbiotic.
the atmosphere and assimilate it with an organic molecule (Figure 9.2). Eventually these organic molecules with nitrogen incorporated into their structure are distributed throughout the food chain as the algae are consumed by higher life forms.
The movement of nitrogen and its changes in oxidation states from the atmosphere to living organisms to the activated sludge process and its return to the atmosphere is thewastewater nitrogen cycle(Figure 9.3). This cycle incorporates the following critical nitrogenous compounds: molecular nitrogen, amino acids, proteins, urea, ammonium ions, ammonia, nitrite ions, andnitrate ions. Amino acids and proteins are organic forms of nitrogen. Molecular nitrogen, ammonium ions, ammonia, nitrite ions, and nitrate ions are inorganic forms of nitrogen.
-
- c-
Continue reading here:Nitrogen Assimilation 79 TABLE 102 Molecules and Ions Providing the Nitrogen Nutrient
Was this article helpful?
